Subheadings
– Oxygen transport and storage
– Oxygen consumption and delivery
– Oxyhaemoglobin dissociation curve
– Hypoxia
– Carbon dioxide
Oxygen transport and storage
The transport of oxygen flows along a cascade of pressure, from a higher pressure source (atmosphere, 21 kPa) to a lower pressure destination (mitochondria, <5 kPa). At each stage in this cascade there is a subsequent decrease in the pressure of oxygen until it reaches the mitochondria. The alveolar-capillary membrane is extremely thin in order to facilitate rapid gas exchange.
Oxygen cascade:
- Atmosphere; 21 kPa
- Alveoli, 13.3 kPa
- Arterial blood, 13 kPa
- Mitochondria, <5 kPa
- Venous blood, 5ish kPa
The oxygen cascade follows Fick’s Law; the rate of diffusion of a substance (O2 in this case) is directly proportional to the concentration gradient (or partial pressure, for gases). So anything that affects the partial pressure of oxygen can change the cascade and, consequently, amount of oxygen being delivered from the atmosphere to the tissues. This is why high altitudes can result in hypoxia, because the atmospheric partial pressure of oxygen is markedly reduced compared to sea level.
As oxygen is taken up by the various tissues, the partial pressure subsequently drops as the oxygen passes from arterial, to capillary, to venous vasculature. The initial drop in partial pressure from the atmosphere to the arterial blood is due to various degrees of water vapor, carbon dioxide mixing, physiologic shunt, and anything else that may affect diffusion across the alveolar/capillary membrane.
In the blood, 98% of O2 is bound to haemoglobin (Hb), with 2% unbound in the plasma (PaO2 on a blood gas sample). The concentration of O2 in the plasma is directly proportional to the partial pressure of oxygen (Henry’s Law). Oxygen diffuses from the Hb, into the plasma, and into the target tissues, in accordance with the haemoglobin-oxygen dissociation curve (more below).
Storage of oxygen is distributed across a number of compartments in the body:
- Plasma (Hb + unbound) – approx 850ml
- Lungs (as FRC) – 270ml (or 3ml/kg)
- Myoglobin (in the muscle) – approx 250ml
- Total O2 storage – approx 1400ml
Anything that affects any one of these may influence O2 storage capacity; anemia, blood loss, lung disease, sarcopenia, rhabdomyolisis etc.
Haemoglobin (Hb)
Red blood cells are essentially bags for carrying Hb. HbA is the most common type of Hb (98%). Each Hb contains 4 subunits, or globin chains; 2 alpha and 2 beta chains. Each of these subunits/globin chains has its own haem group, which contains an iron ion centre. Iron (Fe2+) in the reduced, ferrous state can bind O2 to become the oxidized, ferric state (Fe3+).
Binding of O2 is co-operative; that is, each O2-bound subunit increases other subunits’ affinity for O2 binding, by changing the conformational shape of the adjacent globin chain to more easily bind the next O2 molecule.
As for myoglobin, it exists only in muscle, and has only one haem group to bind O2. The role of storage in the muscle is to provide quick O2 to the mitochondria when demand is high. Diving mammals such as whales have particularly high levels of myoglobin. Serum myoglobin levels in patients can be markedly raised in instances of muscle damage such as trauma or rhabdomyolysis.
Pathological forms of Hb
There are abnormal forms of Hb that are either inborn or can be induced.
- Methaemoglobinaemia:
- Occurs when Hb is oxidized by means other than oxygen binding, taking the haem group from ferrous (Fe2+) to ferric (Fe3+) state, resulting in MetHb.
- Normally there is <1% of MetHb in health, and glutathione or NADH serve to reduce oxidizing agents or MetHb to avoid pathologically raised levels of MetHb.
- Two big problems of MetHb are:
- 1) oxygen cannot bind to MetHb, causing a functional anemia.
- 2) the oxidized MetHb causes a left shift on the dissociation curve, reducing the ability of Hb to offload O2 to the tissues.
- Causes include failure of the reduction system (eg low glutathione) or agents such as nitrites, nitrates, prilocaine, and some antibiotics.
- Treatment is high-flow oxygen, and methylene blue for severe cases.
- Carboxyhaemoglobinaemia:
- Occurs when carbon monoxide binds to Hb to produce COHb.
- Normally, COHb is <2% or up to 5-10% in heavy smokers. Carbon monoxide has around 250x the affinity for Hb that oxygen does, and so competitively binds tightly to Hb.
- The treatment is to competitively outbind CO with high flow, high pressure O2, sometimes in a hyperbaric environment,
- Cyanohaemoglobinaemia:
- CyanoHb forms when exposed to cyanide. This binds irreversibly to Fe2+ causing a functional Anaheim a that cannot be reversed. It also inhibits the electron transport chain so that mitochondria can’t utilize O2, causing lactic acidoses from anaerobic metabolism.
- Sickle cell disease
- is an inborn abnormality of haemoglobin structure and function, resulting in rigid, ‘sickle’ shaped red blood cells that can cause acute complications such as sickle cell crises.
A-a gradient
The difference in PO2 between the alveoli and arterial blood is represented by the alveolar-arterial (A-a) gradient. A normal A-a gradient is less than 2 kPa, but can increase with age and different pathologies. Some factors that can affect the gradient and subsequent level of O2 in the blood include:
- Diffusion across the alveolar-capillary barrier: Normally the pulmonary blood has ample time passing the alveoli to allow adequate oxygen diffusion to reach equilibrium with the alveolar PO2. However, if there is anything slowing down or impeding diffusion this can reduce the arterial PO2, as the blood does not have adequate time to be fully oxygenated whilst passing the alveoli. Causes include edema, consolidation, inflammation etc.
- V/Q mismatch: physiologic mismatch reduces PaO2 by 2-5% versus PAO2, giving a kPa of 13 versus 13.3.
Oxygen delivery and demand
VO2 is the global consumption of oxygen by the body per minute.
In an average 70kg man, this equates to around 250ml/min at rest, and increases with exercise or metabolic demand until a maximum O2 consumption rate is reached (VO2 max). VO2 max is likely limited by diffusion capacity of O2 being reached in the muscle’s micro-circulation.
A rise in VO2 can occur from inflammation, sepsis, pyrexia, burns, trauma, seizures; anything that increases the body’s oxygen demand.
DO2 is the volume of O2 that can be delivered to tissues from the lungs per minute.
The anaerobic threshold is reached when DO2 is at a maximum. At rest, DO2 is higher than Vo2 (eg 1000ml/min vs 250ml/min), meaning there is a good degree of buffer capacity available. If VO2 rises, or DO2 falls (eg hypotension, hypoxia, anemia), then there will be a higher oxygen extraction ratio (OER).
Organs such as the heart have a high OER (0.6) meaning they are very susceptible to ischaemia (eg if ↓ coronary artery perfusion from atherosclerosis or acute MI), as they need to extract a proportionately large amount of oxygen from the supply provided in the blood.
Oxygen-haemoglobin dissociation curve
The sigmoid shape of the curve indicates that only a small change in the partial pressure of oxygen (kPa on the horizontal axis) is required to markedly change the oxygen saturation of the haemoglobin (% on the vertical axis).
Venous oxygen tension is around 5.3 kPa, giving an O2 saturation of around 75%. For arterial blood, oxygen saturation falls precipitously below an arterial partial pressure of oxygen (PaO2) of around 8 kPa. Normal PaO2 is around 11-13 kPa (remember that alveolar partial pressure of O2 is around 13), giving a comfortable safety margin of O2 saturation. O2 saturation is around 100% at a PaO2 of 13, and so greater increases in inspired O2 will not saturate any more haemoglobin, but will increase the PaO2 of oxygen dissolved in blood.
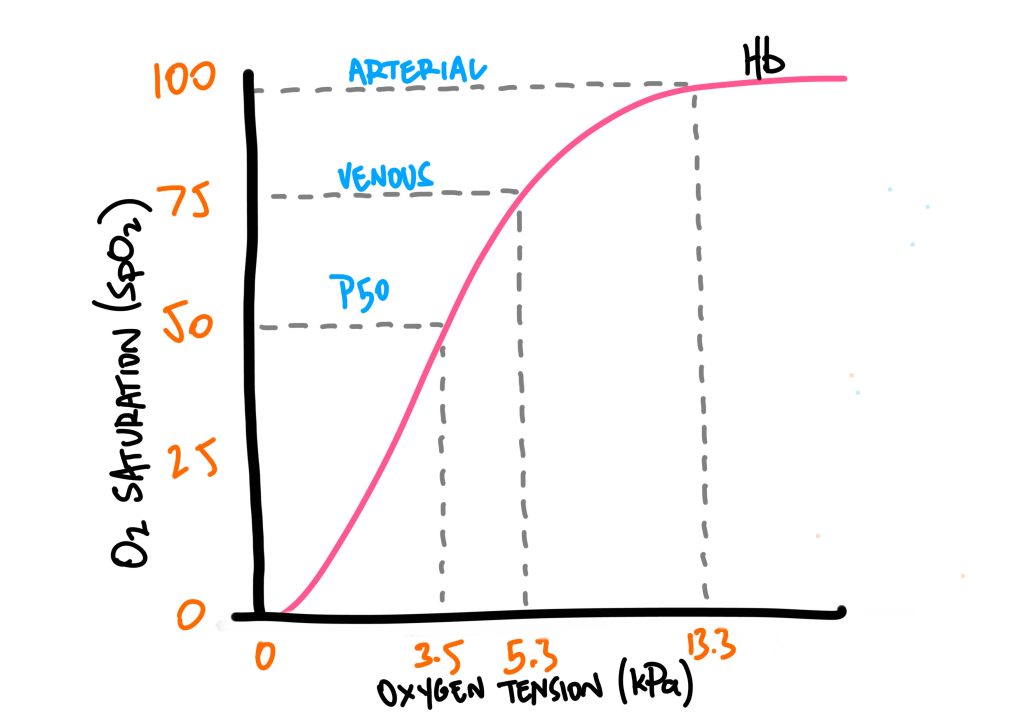
The sigmoid curve can shift leftward or rightward, depending on different conditions.
A rightward shift means that Hb has less affinity for O2, and offloads it more readily. As an example, a rightward shift may mean that a PaO2 of 5.3 kPa is required for 50% SpO2, versus a PaO2 of 3.5 kPa. This means that there is less affinity for O2, and so more will be offloaded. This is useful around areas where O2 demand is high, such as metabolically active tissues that need more oxygen to continue electron transport chain metabolism and energy production.
Factors that cause a rightward shift are summarized by the acronym CADET:
- C – carbon dioxide
- A – acidosis (↓pH)
- D – DPG
- E – exercise
- T – raised temperature
The Bohr effect describes the process where metabolically active tissue produces H+, CO2, and heat. Looking at the above, we can see that these all cause a rightward shift of the sigmoid curve, which leads to increased offloading of O2 from Hb to be taken up by this tissue.
A leftward shift means Hb has a higher affinity for Hb. This is caused by the opposite; reduced PCO2, alkalosis, reduced DPG, and reduced temperature. This typically occurs closer to the pulmonary circulation, so that O2 can be taken up by Hb during gas exchange.
Hypoxia
Hypoxia can occur by the breakdown of a number of different components along the respiratory system. The ingredients required upon for adequate oxygenation through the body include:
- Adequate oxygenation – there must be enough oxygen from the lungs to the arterial blood
- Oxygen carrying capacity – by the hemoglobin primarily
- Sufficient cardiac output – to transport oxygen around the tissues
- Mitochondrial function – to utilize the oxygen in the tissues
The ways that these components may fail include:
- Hypoxaemic hypoxia – ie low PaO2 (≤8 kPa will see a precipitous drop in SpO2) – see below for specific issues that can lead to hypoxaemic hypoxia.
- Anaemic hypoxia – ie reduced O2 carrying capacity. Can occur from reduced Hb, or a functional anemia such as carboxyhaemaglobinaemia and methaemoglobinaemia.
- Stagnant hypoxia – if the blood is not reaching the target tissues eg cardiogenic shock, acute limb ischemia, type 2 MI
- Cytotoxic hypoxia – when mitochondria fail to utilize the O2 eg in severe sepsis, cyanide poisoning
Hypoxaemic hypoxia tends to be the most common cause of hypoxia, and can occur from a number of ways:
- Normal A-a gradient
- Hypoventialtion – if ventilation is reduced, PAO2 is reduced, leading to a fall in PaO2 and subsequent hypoxaemic hypoxia. Causes can include CNS depression, peripheral nervous system dysfunction (spinal cord injury, GBS, MND), NMJ dysfunction (paralysis, MG), muscular weakness, and chest wall abnormalities.
- Low FiO2 – low inspired O2 tension (eg at high altitude).
- Raised A-a gradient
- Diffusion limitation – anything that affects the diffusion of O2 across from the alveoli to the arterial blood. Eg thickened alveolar-capillary barrier (oedema, inflammation) or reduced alveolar surface area (emphysema).
- Shunt – when the alveolus is perfused but not ventilated, V/Q ratio = 0.
- V/Q mismatch (of which the most common is low V/Q ratio).
Carbon dioxide transport
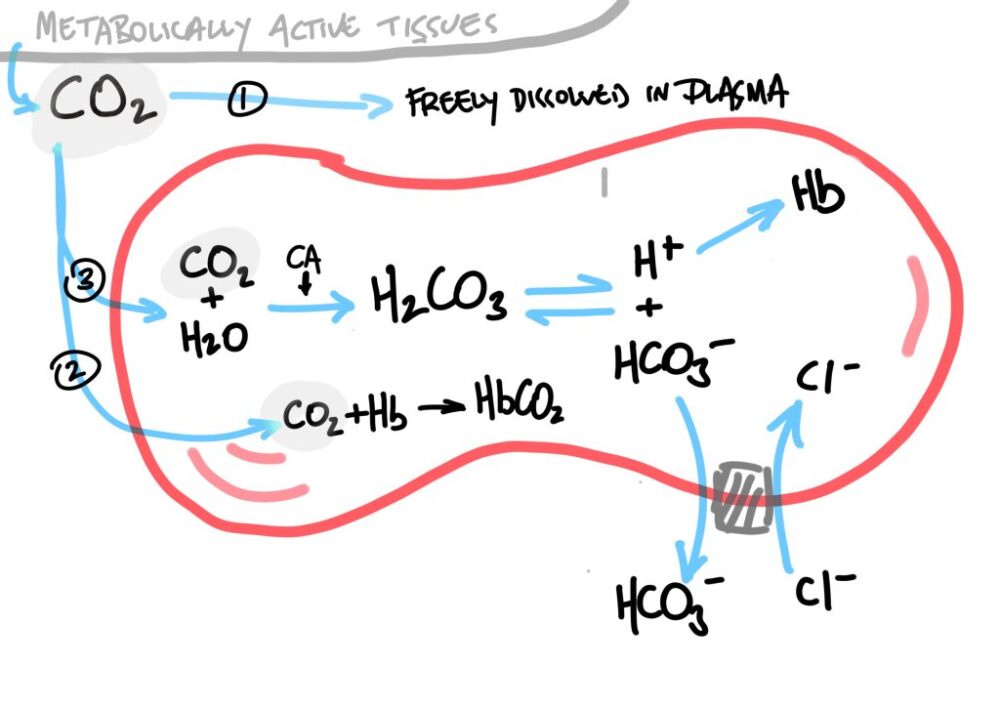
Carbon dioxide is transported by 3 different means.
- 10% as freely dissolved in the plasma
- 20% as carbamino compounds – CO2 binds to sites on hemoglobin, binding more easily to deoxyhaemoglobin than oxygenated hemoglobin
- 70% as Bicarbonate. CO2 can freely diffuse from tissues, into plasma, and into RBCs. Here, CO2 combines with H2O, and via carbonic anhydrase, forms H+ and HCO3-. The hydrogen ions are buffered by binding to sites on hemoglobin, whereas the bicarbonate is exchanged out of the RBC for a chloride ion into the RBC (known as chloride shift). This maintains the electro-neutrality of the inside of the RBC, whilst allowing the equation of CO2 and H2O into H+ and HCO3- to continue, due to this being an equilibrium reaction.
Remember the Bohr effect; that the increase in PCO2 and/or decrease in pH will lead to a rightward shift of the oxygen-haemoglobin dissociation curve, causing a reduced affinity for O2 and promoting offloading to the adjacent tissues.
This compliments the Haldane effect; where deoxygenated hemoglobin binds more easily to CO2, so that it can now be transported to the lungs, where it can diffuse down its concentration gradient out of the blood into the alveoli, to be ventilated off into the atmosphere.
In the lungs, O2 binds to the deoxyhaemoglobin, prompting release of H+ to bind with HCO3 and be converted to H2O and CO2, as well as the direct release of CO2 from carbaminohaemoglobin.