Subheadings:
– Ventilation-perfusion (V/Q) relationship
– Dead space
– Shunt
– Pulmonary circulation
Ventilation Perfusion (V/Q) relationship
Ventilation (V) refers to the movement of air into an alveolar space, whilst perfusion (Q) is the flow of blood to an alveolar space. Ventilation and perfusion are two partners of a relationship that is required for gas exchange. There are numerous things that can affect either component and potentially cause a mismatch to occur.
Gravity
Gravity impacts blood flow (perfusion) and alveoli (ventilation). Let’s look at how gravity affects each of these in turn.
Perfusion
Perfusion increases linearly from the apex to the base of the lungs, where blood pools most due to gravity. The differences in regional pulmonary artery pressure (PAP) is largely mitigated by adequate cardiac output, which ensures an acceptable mean pulmonary artery pressure (MPAP) throughout the entire lung vasculature, with blood distributed more evenly through the lung; this is more so during exercise, for example, when cardiac output increases furthermore.
In addition to affecting pulmonary blood pooling, gravity exerts a stretch on the apical alveoli as the lungs hang down, pulling the tissue in the apex open. In theory, the apical alveoli, stretched open, can exert a compressive pressure on the surrounding pulmonary vascular supply, causing vessel collapse and reduced perfusion. However, in normal lungs, alveolar pressure does not normally exceed pulmonary arterial pressure (also known as West Zone 1). Situations where this may occur include significant shock, with reduced pulmonary arterial pressure, or increased alveolar pressures, eg from invasive ventilation. Conversely, at the bases, the lung tissue is under little pressure, and so surrounding vessels have plenty of room for blood flow.
Ventilation
Ventilation is also affected by gravity, with ventilation increasing linearly from base to apex. This is because the sheer weight of lung tissue hanging down exerts tractional force on the airways, with the alveoli in the apex being stretched open the most. Already stretched open, the alveoli in the apex sit on a flatter curve, further along on the compliance curve, and don’t open much more with increased pressure. In contrast, the basal alveoli are most compressed, but most compliant; during tidal inspiration their volume will increase much more than at the apex, hence they sit on a steeper part of the compliance curve.
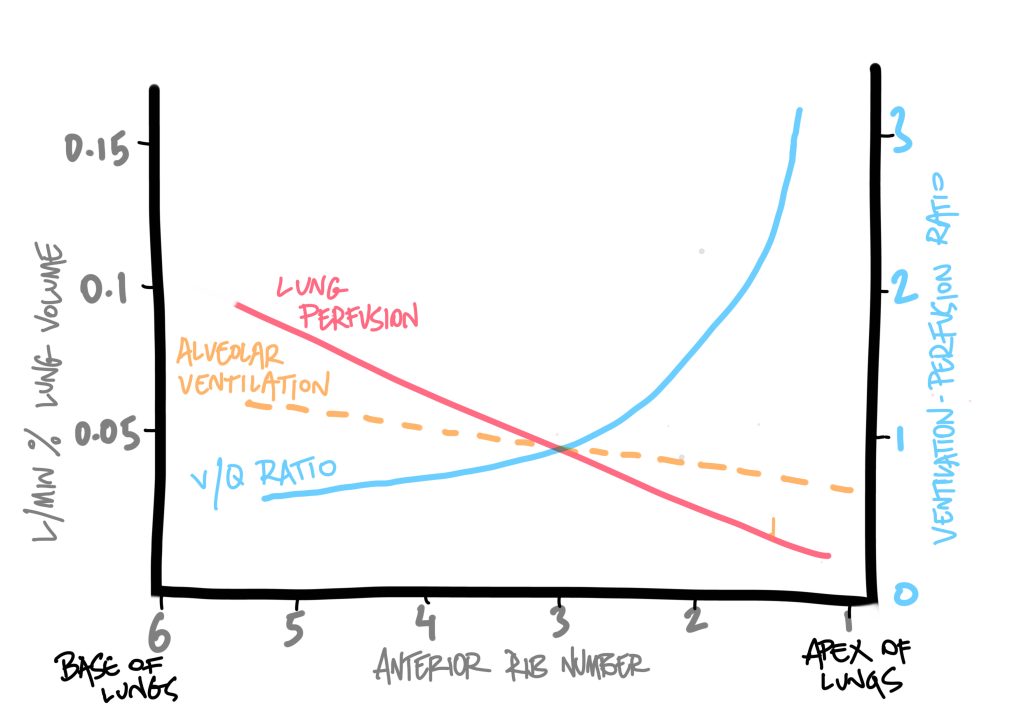
Hence, ventilation and perfusion are both, in general, greater at the bases of the lungs than at the apices, due to gravity. Proportionately, perfusion is more greatly affected than ventilation across this apex-to-base gradient.
Position can influence perfusion, for example in a lateral position with the lowermost (dependent) lung receiving the greatest perfusion. Proning takes advantage of this gravity dependant nature, by positioning the best ventilated areas of lung in the lowermost position, to ensure increased perfusion.
In the example of positioning, imagine a severe right sided pneumonia. By positioning the patient onto their left side, with their better lung down, we can take advantage of gravity to increase perfusion and compliance in the better, less pathological lung tissue and, hopefully, improve ventilation and oxygenation.
Ventilation-perfusion ratio (V/Q)
Ideally, ventilation (V) would perfectly match perfusion (Q); no wasted respiratory effort or redundant blood flow. In theory, the V/Q ratio would be 1, as ventilation and perfusion would be perfectly matched.
But even in normal physiology, this is not the case. Perfusion occurs at roughly 5L/min, whilst ventilation is roughly 4L/min; this means there is, globally, more blood flow than ventilation across the lungs.
V(4) / Q(5) = 0.8
This means that in health, there are more areas of underventilated/over-perfused lung, causing this mismatch to occur. Although this is a global lung average, as we have seen above, there is considerable regional difference. For example, at the apices, where ventilation is greater than perfusion, the V/Q ratio is closer to 3, whilst at the bases, where perfusion is greater than ventilation, V/Q ratio is closer to 0.6.
What do these numbers mean?
- V/Q = >1.
- When there is more dead space (ventilation with relatively less perfusion).
- V/Q = <1.
- When there is more shunt (perfusion with relatively less ventilation).
In pathology, most V/Q mismatch is <1, due to the lung tissue being more commonly affected by states such as pulmonary oedema, pneumonic consolidation, mucous plugging, effusions, etc. These are states where ventilation is predominantly affected, resulting in perfused areas of lung not taking part in gas exchange. When this is extreme enough, this kind of V/Q mismatch behaves like shunt physiology.
Let’s look at dead space and shunt independently.
What is dead space?
This describes areas of the lungs that are ventilated, but do not take part in gas exchange. Physiologic dead space encompasses two sub-types of dead space:
- Anatomical dead space: this is the unmodifiable dead space of the conducting zone of the respiratory system. For example, the air present within the main bronchi at maximum inspiration, will not be able to reach the respiratory zone to take part in gas exchange.
- Alveolar dead space: these are areas within the respiratory zone, where alveoli are unable to take part in gas exchange, such as poorly perfused areas of the lung. Alveolar dead space is also termed ‘pathological’ dead space and can be influenced by disease states.
Anything that reduces alveolar perfusion, or increases areas of under-perfused alveoli, will increase dead space.
- PEEP at high enough levels will lead to higher pressures within the alveoli, and compress the alveolar capillaries, preventing perfusion. High PEEP can also reduce venous return due to low pressures in the pulmonary venous system (5-15mmHg), and reduce perfusion.
- Low pulmonary artery pressures or impaired pulmonary artery blood flow
◦ Right ventricular dysfunction will cause reduced perfusion of apical alveoli.
◦ Pulmonary artery obstruction (embolus/thrombus/fat/gas) will also reduce perfusion and increase dead space. - COPD leads to the destruction of alveolar septa, with reduced area for gas exchange, and so more alveolar dead space.
- Upright posture causes blood pooling to the base of the lungs due to gravity, with reduced perfusion (and therefore more dead space) in the apices, which are well ventilated.
What is shunt?
Shunt describes the passage of deoxygenated blood from the right side, to the left side of the body, without undergoing oxygenation. Basically, deoxygenated blood reaching the left side of the heart and body.
Physiologic shunt (analogous to physiologic dead space)
- Anatomical: venous drainage from cardiac veins and bronchial veins into the right side of the heart.
- Functional: some pulmonary blood passes through poorly ventilated areas in the lung bases, therefore not fully oxygenated.
Physiologic shunt reduces global gas exchange by around 2-5%.
Pathological shunt
- Cardiac / large vessel: eg VSD, PDA, pulmonary AVM
- Intra-pulmonary: by far the most common pathological shunt eg pneumonia, edema, effusion etc.
What is the difference between shunt and V/Q mismatch?
V/Q mismatch, where V/Q <1, refers to perfusion with a relative decrease in ventilation. Mild to moderate V/Q mismatch of this type normally responds to oxygen therapy, by increasing the total fraction (FiO2) of inspired O2 to compensate for the reduced ventilation. This increased FiO2 leads to an increased PAO2 (partial alveolar pressure of O2), a higher pressure gradient at the alveolar-arterial barrier, and greater diffusion of O2 into the blood (with a resulting higher partial arterial pressure of O2, or PaO2).
In pure shunt physiology, increased FiO2 would have no effect, as the blood would be bypassing any ventilated areas of lung, and thus unable to access any of the increased FiO2. If V/Q mismatch is severe enough, this can act like a shunt, with a progressively poor response to oxygen therapy. At this point, the different terminology of shunt and V/Q mismatch are largely academic, as they are clinically the same problem.
Causes of low V/Q ratio
This is anything that reduces ventilation relative to perfusion (ie shunt physiology), and so is commonly encountered in clinical practice.
- Fluid within alveoli eg Pulmonary oedema, pneumonic consolidation
- Ageing and emphysema (due to reduced surface area)
- Pneumothorax
- Upper airway obstruction
- Obesity, poor posture, pregnancy (due to greater atelectasis)
Causes of high V/Q ratio
Anything that reduces perfusion relative to ventilation (ie dead space physiology)
- Pulmonary embolism
- Reduced RV stroke volume eg due to RV infarction
- Hypovolaemia
- Excessive PEEP (compresses pulmonary blood vessels)
Pulmonary circulation
Compared to the systemic circulation, the pulmonary system is a low pressure, low resistance, high flow circulation system. It has to be perfused with around 5L/min by the relatively less muscular right ventricle, with a systolic pressure of around 15-30 mmHg. Mean pulmonary artery pressure is around 10-20 mmHg, with ≥25 mmHg defined as pulmonary hypertension.
If pressures were higher, there would be risk of transudative pulmonary edema across the thin capillary membranes into the alveoli. It’s a balance between a thin barrier for best gas exchange, and high enough pressure to perfuse the lungs.
A number of things can affect the pulmonary vascular resistance (PVR) of this system
- Pulmonary artery pressure. Strangely, a raised MPAP results in a reduced PVR. This is primarily by increased recruitment of capillaries (therefore greater surface area to reduce PVR), and the high distensibility of pulmonary vessels.
- Lung volume also affects PVR. Extra-alveolar blood vessels, not exposed to alveolar pressures, are pulled open on increased lung volumes, and so PVR drops. When lung volumes drop, then these vessels close down due to unopposed elasticity, and so PVR rises.
- Hypoxic pulmonary vasoconstriction (HPV). Opposite to the systemic circulation, regional hypoxia causes pulmonary vessels in that area to constrict, with the aim of diverting blood to better oxygenated areas of the lungs. This diversion will improve V/Q mismatch, but also raises PVR. Oedema, pulmonary emboli, and cardiovascular disease can increase HPV and, in turn, PVR.
- COPD causes chronic HPV due to poorly ventilated areas of lung, and this raises PVR which can, in turn, cause pulmonary hypertension.
- Cardiovascular disease may lead to reduced LVF and upstream pressure increases in the pulmonary vascular system (due to increased afterload). This can lead to pulmonary hypertension and, eventually, cor pulmonale (dilatation, hypertophy, structural change of the right ventricle).