Lung volumes and capacities
Lung volumes refer to the volume of gas within the lungs at different times during the inspiratory cycle. Normal tidal breathing, which is the respiratory pattern when at rest, has a volume (tidal volume) of around 500ml, including both inspiration and expiration. At end inspiration on tidal breathing, we still have the ability to inhale a greater volume (inspiratory reserve volume) of around 2500ml. At the end of tidal expiration, we can still force out a volume (expiratory reserve volume) of around 1500ml. At the end of maximal exhalation, there is still a volume (residual volume) of around 1500ml, otherwise our lungs would be totally collapsed down. These are all approximate ballpark figures, and are affected by age, posture, habitus, pathology etc.
Lung capacities are the sum of 2 or more lung volumes. The entire capacity of all these volumes is around 6000ml (total lung capacity). If we exclude the part we can’t alter (residual volume) then the capacity is around 4500ml (vital capacity). The inspiratory capacity is around 3000ml, and is the capacity of gas that can be maximally inhaled after a tidal exhalation (at the end of a normal breath).
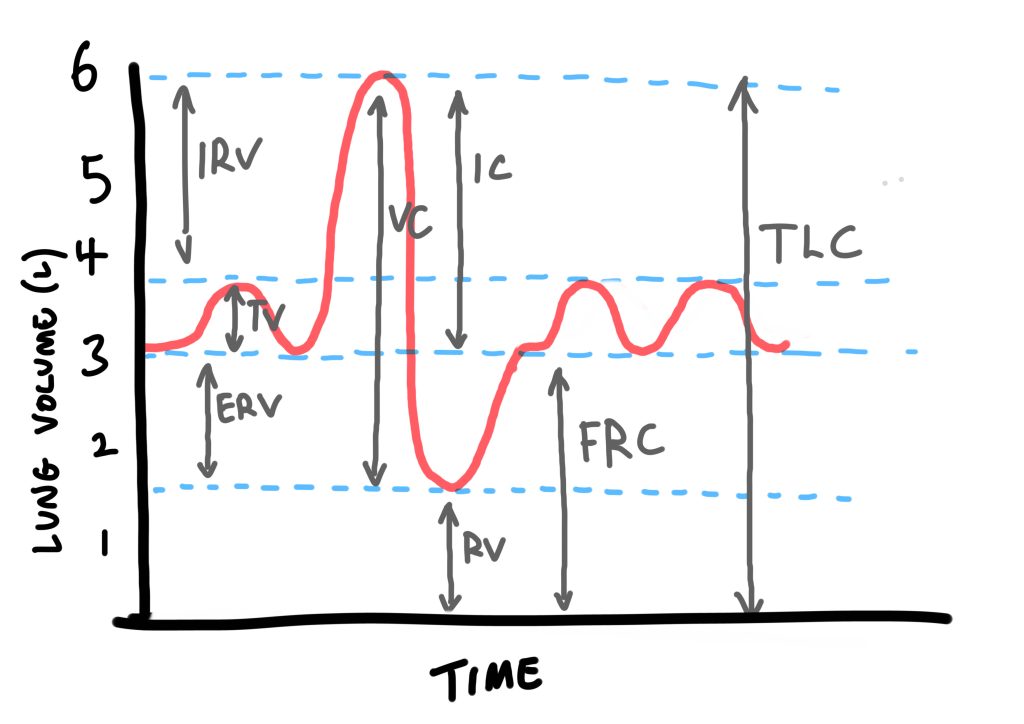
4 lung volumes
- TV – 500ml
- ERV – 1500ml
- RV – 1500ml
- IRV – 2500 ml
4 lung capacities
- TLC – 6000ml
- VC – 4500ml
- FRC – 3000ml
- IC – 3000ml
Functional Residual Capacity (FRC)
Clinically, the functional residual capacity (FRC) is useful to understand, and is the sum of ERV and RV; essentially, the volume of air left in the lungs at the end of a normal, passive exhalation. It acts as the reservoir of oxygen that the body can use when not ventilating.
It is the point in the respiratory cycle where the inward, elastic recoil forces of the lung tissue are at equilibrium with the outward forces of the chest wall, which want to spring open. In other words, the position and volume at which the lungs find themselves when at rest, after end-passive expiration.
FRC is characterized by a number of important functions:
- It acts as an O2 reservoir. In a clinical setting, pre-oxygenation (more below) with 100% FiO2 can saturate this reservoir with O2, and wash out the nitrogen that would otherwise make up most of the volume in the lungs.
- Ongoing gas exchange. FRC also allows oxygenation of the pulmonary blood through the whole respiratory cycle; If FRC didn’t exist, we would be at risk of intemittent hypoxia during expiration, because there would be no air in the lungs at end of expiration.
- Minimise work of breathing. FRC is the point where compliance is greatest, and the work required to open the lungs lowest; the lung tissue is already open, and so small changes in pressure result in proportionately larger changes in lung volume.
- Minimise RV afterload. This is because at FRC, the pulmonary vascular resistance (PVR) is at a minimum. Conversely, at end inspiration, the inflated alveoli can compress capillaries and raise PVR, whereas at end expiration, some alveoli may collapse and cause hypoxic pulmonary vasoconstriction, also causing raised PVR. FRC is a state that exists between these two extremes.
- FRC prevents atelectasis and V/Q mismatch. Without FRC, the lung tissue would collapse in, and there would be blood flowing to the lungs without any gas exchange taking place (shunt).
Reductions in FRC
Can be caused by anything that reduces overall lung volume, such as lying supine (reduction by around 20% compared to sitting upright) and raised intra-abdominal pressure. Anaesthesia also causes a reduced FRC (by around 20%), likely due to reductions in muscle tone and physiologic PEEP. In addition, many lung disease states such as fibrosis, pulmonary oedema, ARDS, and atelectasis causes reduced FRC; essentially, any disease that reduces lung volume.
Increases in FRC
FRC can be helpfully increased by PEEP, which will stent open more airways and decrease atelectasis. This can be done with high flow nasal oxygen and, more effectively, CPAP (continuous positive airway pressure), via a mask/hood/ventilator.
Emphysema and ageing increases FRC, by way of reduced elastic lung tissue —> more open airways —> greater lung volumes at rest. Asthma anlso increases FRC pathologically by way of increased air trapping and high intrinsic PEEP. Patients suffering a COPD exacerbation will often used pursed-lip breathing which, on expiration, provides higher levels of auto-PEEP, in order to reduce airway collapse.
Clinically helpful ways to increase FRC include:
- Treat any underlying respiratory pathology
- Optimise position – this usually means ramping or sitting the patient up
- Ensure patent airways eg treat bronchospasm
- Use PEEP – eg HFNC/mask/hood/invasive ventilation
Pre-oxygenation
As noted above, pre-oxygenation is the process of saturating FRC with O2, and displacing as much nitrogen as possible to facilitate a greater concentration of O2 in the lungs, all with a view to delaying hypoxia for as long as possible during a period of apnoea.
FRC is ball-parked at around 3000ml (ERV of 1500ml + RV of 1500ml).
As a worked example below:
- More accurately, FRC is calculated at around 30ml/kg
- For a 70kg individual, this will be 2100ml/kg
- The fraction of oxygen in atmospheric air (FiO2 – fraction of inspired oxygen) is around 0.21. By the time it reaches the lungs, it drops to around 0.13 in the alveoli (FAO2 – fraction of oxygen in alveoli), due to mixing with expired CO2, water etc.
- In an FRC of 2100ml, this means around 250ml is oxygen (0.13 x 2100 = 273).
- Oxygen consumption is around 3ml/kg/min (lets say 250ml per minute) at rest, so this reservoir will last approximately 1 minute before hypoxia occurs.
- If we pre-oxygenate with 100% FiO2, we can achieve an FAO2 of around 0.9 (versus 0.13)
- This increases our FRC oxygen reservoir to nearly 2000ml (0.9 x 2100 = 1890ml).
- Theoretically, this gives a reservoir of oxygen that allows around 7-8 minutes of apnoea time (assuming 250ml O2 consumption per min) before hypoxia occurs.