Having used NIV successfully many times, I was taken aback when I experienced my first treatment failure with it.
My patient was rushed into resus from the reception, with oxygen saturations reportedly 40% on room air, brought up to >90% with a non-rebreathe mask. An ABG showed respiratory acidosis, and nebulisers and steroids were started whilst setting up the NIV machine. A repeat ABG after this round of medical treatment showed worsening hypercapnic respiratory failure.
NIV was started, achieving good oxygenation, tidal volumes, and respiratory rate. Despite this, the patient deteriorated with worsening consciousness, PaCO2, pH, and PaO2. After repeat CXR to screen for new pneumothorax, optimising NIV settings, and further medical management, it became clear that this was not reversible. Discussions were had with colleagues and family, and the decision was made that we would move to prioritising the patient’s comfort.
I was surprised to learn that, in fact, around 1/4 of NIV treatments fail, usually proceeding to either intubation or palliation. Seeing a patient deteriorate with worsening consciousness, PaCO2, pH, and PaO2, despite the NIV machine functioning as required, subsequently made me question more about the underlying principles of NIV.
This is not a wholly encompassing NIV tutorial (that would be huge), but addresses some key areas of the topic, as well as my own questions.
What is NIV?
Non-invasive ventilation (NIV) is an umbrella term for therapies that assist oxygenation and ventilation but, unlike invasive ventilation, do not use intubation.
The mechanism of NIV is delivery of positive pressure external air/oxygen flow to the lungs.
There are two types of NIV:
Continuous Positive Airway Pressure (CPAP)
Quite simply, CPAP is a constant level of pressure this is continuously delivered to the patient throughout the respiratory cycle. If we take atmospheric pressure to be 0 cmH2O, CPAP generally delivers positive pressures of between 3-10 cmH2O; this is sometimes likened to the feeling of sticking your head out of a fast moving car whilst breathing.
CPAP only delivers one pressure, and so is appropriate for treating hypoxaemic respiratory failure, where oxygenation (not ventilation) is the primary problem. CPAP/PEEP/EPAP are all analogous to each other.
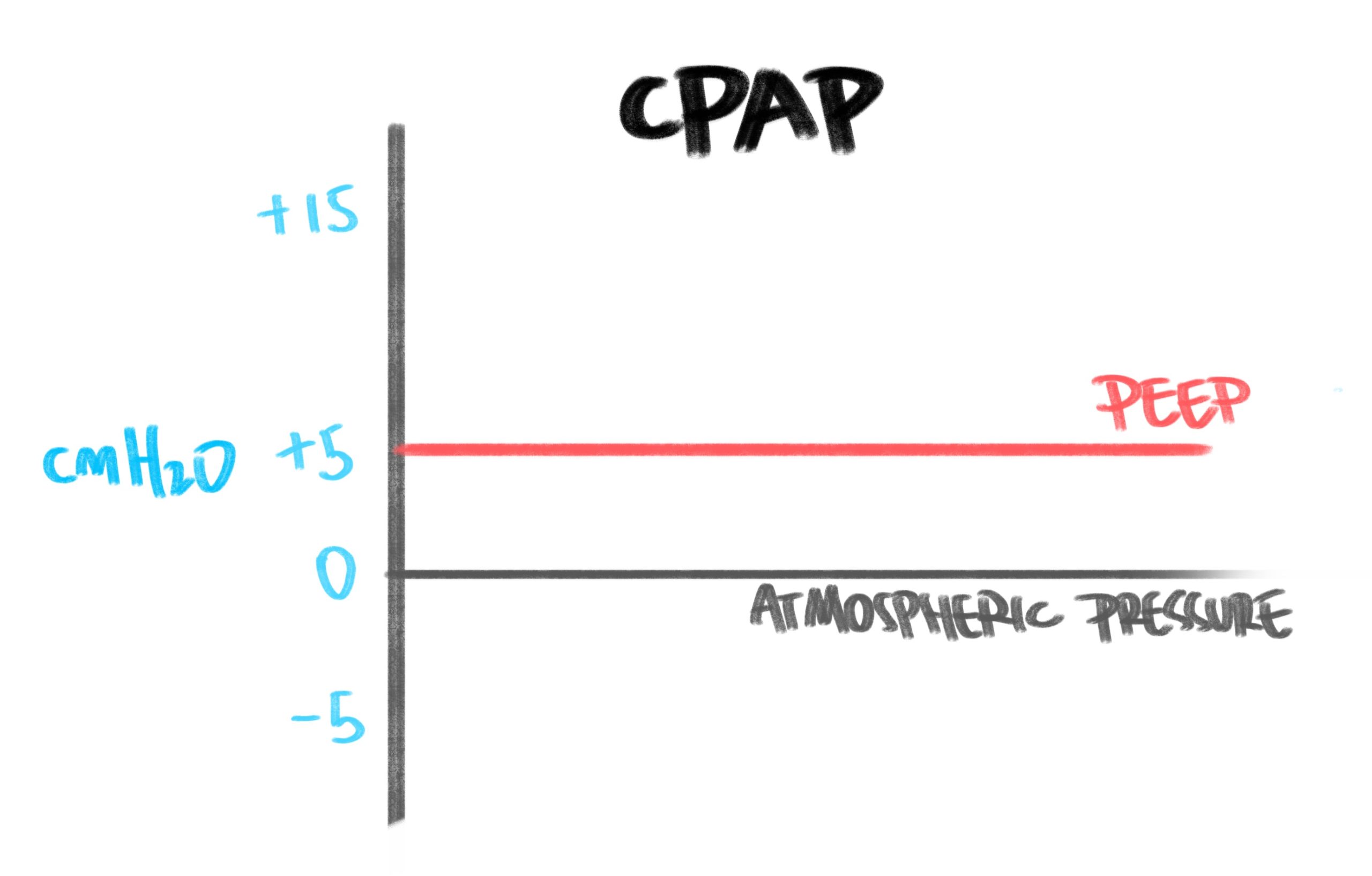
Bi-level Positive Airway Pressure (BiPAP)
BiPAP, on the other hand, adds in a second pressure to provide two levels of pressure; end positive expiratory pressure (EPAP – analogous to CPAP/PEEP) and, additionally, inspiratory positive airway pressure (IPAP – a higher pressure that is delivered for inspiration). EPAP helps with oxygenation, whereas IPAP supports ventilation. The difference between EPAP and IPAP is the driving pressure, which is what assists the flow of air into the lungs during inspiration.
It’s worth noting that EPAP usually won’t use pressures as high as the maximum of those used in pure CPAP, because a driving pressure is required to maintain adequate ventilation. If the pressures get too close to one another, then the difference may be too small, and worsen ventilation.
The BiPAP setting is analagous to pressure support on a ventilator. Because BiPAP delivers a driving pressure, it can be effective in treating hypercapnic respiratory failure and problems with ventilation.
Physiologic benefits of both CPAP
- Increase oxygenation
- Increased recruitment of alveoli to provide greater surface area for oxygenation.
- Increases the oxygen pressure gradient from airways to blood, to deliver higher amounts of O2.
- Reduces V/Q mismatch; recruit alveoli → improved oxygenation of surrounding blood → reduces overall shunt physiology.
- Redistribution of fluid; the positive pressure can ‘force’ oedema or fluid within the lungs back across the alveolar-capillary membrane into the blood.
- Improve work of breathing
- Opening up partially collapsed alveoli brings them to a more favourable point on the compliance gradient (nearer FRC), reducing the negative pressures required to open them up further, thus making inspiration easier.
- Reduces shear damage on alveoli from the collapsing and opening during respiration, instead stenting them open.
- Changes in haemodynamics
- Impedes venous return → reduces preload.
- Reduces left ventricular afterload – acts as a left ventricular assist device.
Specific additional effects of BiPAP
Remember that BiPAP has all the additional features of CPAP, plus:
- Improves ventilation
- Driving pressure opens alveoli and augments inspiratory efforts to improve tidal volumes and minute ventilation.
- Reduces energy/O2 consumption of inspiratory muscles by augmenting inspiratory effort – this can be a profound benefit in exhaustion, critical illness and frailty.
Main use cases
The primary conditions that get the lion’s share of NIV are chronic obstructive pulmonary disease (COPD) and acute cardiogenic pulmonary oedema (ACPO). Other uses (such as obesity hypoventilation syndrome, neuromuscular disease, etc) share some common principles, but won’t be discussed at length here.
Acute Cardiogenic Pulmonary Oedema (ACPO)
Heart failure is characterised by reduced cardiac contractility and cardiac output (specifically, ejection fraction). Additionally, increased end diastolic volume in the left ventricle backs up into the pulmonary vasculature, resulting in fluid/oedema building up in the interstitium and the alveoli themselves; this impedes the alveolar-capillary membrane, increases V/Q mismatch and shunt, and hinders oxygen transport into the body, causing hypoxaemic respiratory failure. Basically, fluid in the lungs impedes the alveolar-capillary interface and reduces gas exchange.
CPAP can help ACPO through:
- Improved oxygenation
- Cardiac unloading.
As detailed above (see Physiologic benefits of both CPAP and BiPAP), CPAP can improve the work of breathing and oxygenation by increased alveolar recruitment, fluid redistribution, and improved compliance. It also alters cardiac dynamics and, thus, cardiac output.
Firstly, lets look at the right side of the heart. Positive pressure ventilation increases intrathoracic pressure, impeding systemic venous return into the right atrium, which reduces preload to the right side of the heart. This increase in intrathoracic pressure also compresses pulmonary vasculature (intra-alveolar vessels), increasing pulmonary vascular resistance (PVR). An increase in PVR results in increased afterload for the right ventricle, as it has to now pump blood forward into the lung vasculatre, against this PVR.
This dual effect of raising RV afterload and decreasing RV preload results in reduced pulmonary venous return and, following that, reduced left ventricular preload.
Next, the left side of the heart. As just mentioned, positive pressure ventilation leads to reduced preload for the left ventricle. Next to discuss afterload.
Afterload for the left ventricle is determined by the pressures in the aorta and systemic vasculature. When we apply positive pressure with CPAP, the intrathoracic pressure is now elevated compared to atmospheric pressure. The aorta and rest of the systemic vasculature reside outside of this zone of newly increased pressure, and so this new pressure difference actually augments the pumping of the left ventricle, effectively reducing afterload and increasing cardiac output.

Credit: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4244615/
An important distinction to make here is between those who are either more preload or afterload sensitive.
- Preload-sensitive: Normal subjects without heart failure, those with right ventricular dysfunction (such as infarction, RVF), or hypovolaemic patients are more preload dependent. Therefore, the effect of CPAP reducing LV preload on the heart may not be outweighed by the reduction in afterload, and may decrease cardiac output overall.
- Afterload-sensitive: Patients with heart failure are usually much less preload dependent due to usually being hypervolaemic. They are also more sensitive to afterload, and so reducing afterload through CPAP in these patients will likely increase cardiac output, without clinically significant negative effects from reduced preload.
Generally, start at a PEEP of around 4-5 cmH2O and titrate cautiously, as required, to 8-10 cmH2O. Whilst doing this, continue medical therapies such as GTN, diuretics, and morphine, in order to treat the acute oedema.
Obstructive airways disease
COPD is far and away the predominant pathology that NIV is used for. Obesity hypoventilation syndrome (OSA) is treated in the same way as COPD regarding NIV. Bronchiectasis with severe broncho-constriction can benefit from NIV under the same parameters as COPD, with the addition of respiratory physiotherapy for secretion management, usually during NIV breaks.
Asthma is more controversial; Josh Farkas and co at EMCrit advocate for NIV in certain circumstances; however UK practice and bodies such as the Royal College of Physicians and the British Thoracic Society say that there is no role for NIV in acute asthma. If asthmatics are reaching such a point of hypoxaemic respiratory failure (eg exhaustion, hypercapnia, respiratory acidosis etc) despite optimal medical therapy, then escalation to intubation is the next step.
The situation is more nuanced in mixed airways disease, or where diagnosed asthma is behaving like COPD eg with heavy smoking history, raised bicarbonate etc. These cases often need more heads together eg with critical care or respiratory physicians.
COPD
The primary issue in acute exacerbations of COPD (AECOPD) is type 2 (hypercapnic) respiratory failure. This is characterised by insufficient alveolar ventilation, leading to poor oxygenation (↓ PaO2), increased levels of blood carbon dioxide (↑ PaCO2), and subsequent respiratory acidosis. If untreated, this eventually leads to respiratory and cardiac arrest.
BiPAP is therefore the choice of NIV in AECOPD; it provides two levels of pressure support to improve alveolar ventilation (the mechanisms discussed previously). Briefly, EPAP helps to improve oxygenation by overcoming auto-PEEP (by stenting open small airways), recruiting more alveoli, and improving compliance. IPAP helps improve ventilation through increased tidal volumes and offloading the respiratory muscles. All of this helps the patient oxygenate, off-load CO2, and resolve respiratory acidosis.
A brief discussion of auto-PEEP / internal PEEP / iPEEP (different terms, all the same thing):
Destruction of lung tissue associated with COPD (emphysema) means that small airways (such as alveolar ducts and respiratory bronchioles) are more prone to collapse, due to loss of alveolar tissue attachments that normally help keep airways open. During an exacerbation, patients’ ventilatory efforts increase and, during expiration, intrathoracic pressure can exceed that of the small airways, causing them to collapse. The alveoli can’t blow off the gas due to the collapsed airways, and so the pressures inside the alveoli increase from this ‘air-trapping’. This results in reduced alveolar ventilation, less oxygen delivery, and more trapping of carbon dioxide, worsening hypercapnia and respiratory failure.
Additionally, the effort required to overcome this auto-peep and enable airflow into the alveoli results in significantly increased work of breathing, and can lead to fatigue and exhaustion.
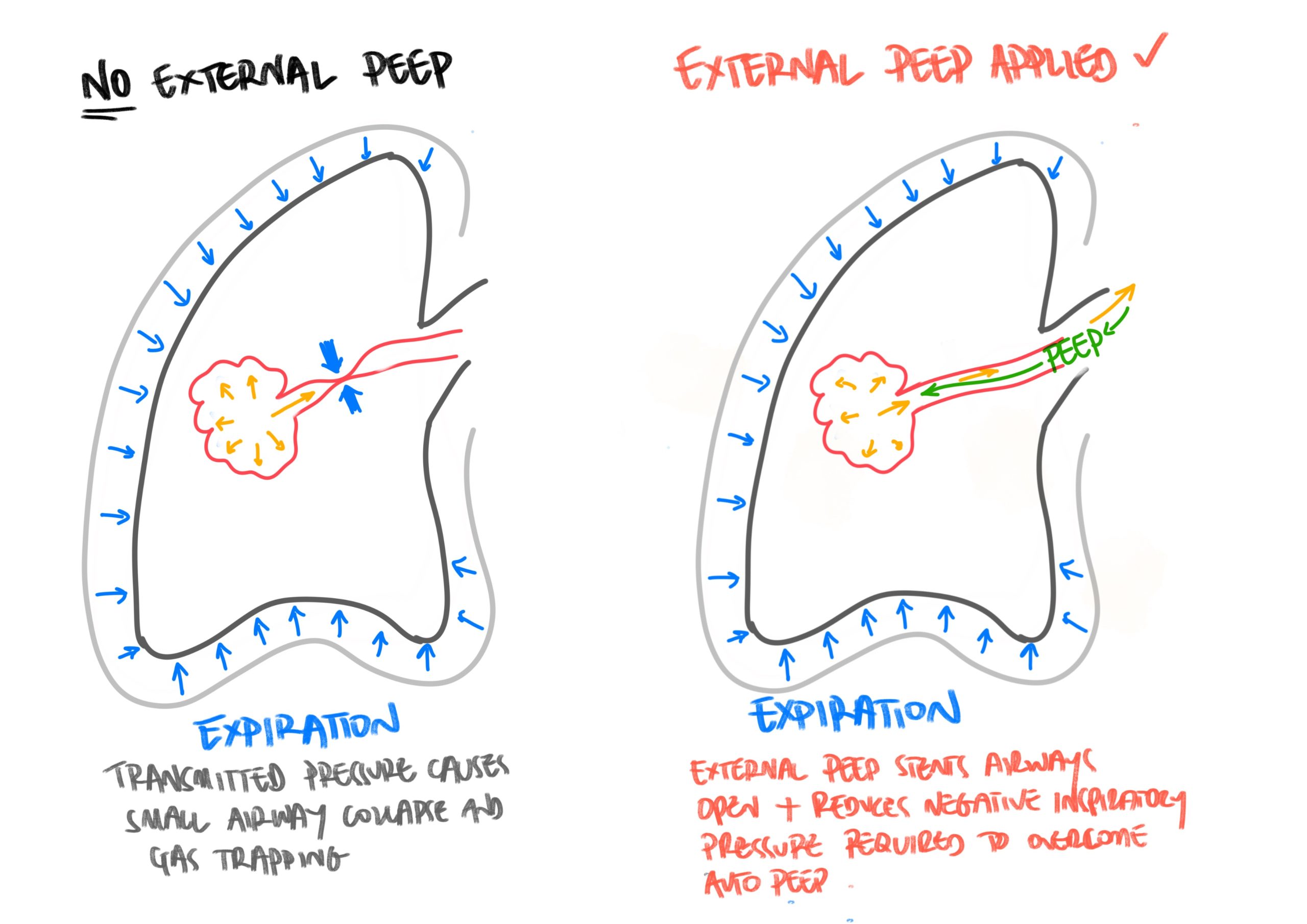
External PEEP (CPAP/PEEP/EPAP) can help stent open the otherwise collapsed airways, which helps the alveoli to blow-off trapped gas and improve alveolar ventilation. However, if external PEEP exceeded internal PEEP, then the alveoli would not be able to ventilate off the carbon dioxide – it would be like trying to swim upstream against the tide. That’s why PEEP should never be too high, or it may exceed internal PEEP (which is often around 12-16 cm H2O).
Auto-PEEP can also occur if expiratory duration is too short, or tidal volumes too high – also termed ‘breath stacking’. In this instance, there is no downstream blockage of air flow out of the alveoli (such as collapsed small airways), and so external PEEP will not help. Strategies to manage this include increasing expiration time and reducing tidal volumes to more appropriate levels.
Indications to start NIV are respiratory acidosis (pH <7.35, pCO2 >6.0) and/or high work of breathing that has not improved despite optimal medical therapy. Aim to get a CXR prior to starting to rule out pneumothorax, but BTS does state to initiate NIV prior to CXR if pH <7.25, then get the CXR asap.
There are some contraindications to consider; anatomic barriers (eg facial burns/trauma/deformity), upper airway obstruction, and aspiration risk (reduced conscious level, recent emesis etc). Also ensure that any pneumothorax is treated with a chest drain to prevent transormation to a tension pneumothorax.
Relative contraindications that require clinical judgement include severe acidosis (may predict NIV failure and may prompt consideration of intubation), agitation (may be managed with careful use of sedation), obtunded GCS (aspiration risk and reduced airway reflexes/ventilation drive). These latter considerations are all patient specific eg a very acidotic patient who would not be a candidate for intubation would likely still warrant trial of NIV.
COPD and pneumonia
As a previous critical care SHO I ran into the idea that pneumonia was a contra-indication to NIV. Whilst NIV is not indicated in standalone pneumonia (drying out mucous and risks of worsening plugging being possible issues), it is an appropriate treatment when COPD is complicated by pneumonia, by utilising the same indications as for non-pneumonic COPD.
BiPAP settings
- Modes
- Spontaneous and timing (S/T) mode: this is the vast majority NIV mode. It will deliver pressure support triggered by patient breaths, but also give extra mandated breaths if the set RR is higher than the patient’s RR.
- There are spontaneous (patient triggered only) and timing (mandated breaths only) modes, S/T is a combination and the most common mode on NIV ventilators.
- The 3 key settings:
- EPAP: start at 4-5 cmH2O. Titrate up if still hypoxic to recruit more alevoli. May require titration up to 8-10 cmH2O if indicated.
- IPAP: start at 12-14 cmH20. Unlike EPAP, aim to titrate up toward 20-30 cmH2O within 10-30 mins, as tolerated. This is to deal with the main problem (ventilation) by proactively increasing the inspiratory support.
- FiO2: the main thing that will help oxygenation is optimising the ventilation settings. However, titrate the FiO2 to achieve target SpO2 of 88-92% (BTS guidelines grade A evidence; regardless of cause of type 2 respiratory failure).
- Other settings (less commonly needed to adjust)
- Breath rate: this gives mandated breaths when not triggered by the patient. eg if patient RR is 8, and breath rate set to 10, the machine will force in 2 additional breaths. Generally aim for at least RR 10.
- Inspiratory time: only for backup breaths if the patient is not triggering.
- Rise time: the time it takes for inspiration to reach full IPAP. If patient is taking rapid, short breaths, then decrease the rise-time so that full IPAP pressure is reached before end of patient inspiration.
A note on settings: in the vast majority of cases, the only really important components to be involved with are EPAP, IPAP and FiO2. If you are playing with breath rate due to low patient RR, they probably have bigger problems that need addressing (?opioid toxicity ?appropriateness of NIV etc).
There will always be a slight leak from the mask. Volumes of up to 60mls leakage are ok, particularly if the expiratory tidal volumes are in the right range. Do not overtighten the mask as it may be uncomfortable and cause pressure damage. Also ensure correct mask size.
Monitoring
- Aim for expiratory tidal volumes (Vte) of approx 4-6ml/kg. This usually equates to around 300-600ml.
- ABGs: aiming for resolving respiratory acidosis
- Patient condition: mental status, work of breathing, RR, how do they look
- SpO2: aiming for 88-92%
NIV Failure
Although the evidence is extremely robust for benefits of NIV in the right patients, there is also a significant failure rate. The BTS National Audit (for all causes of NIV: COPD, neuromuscular disease, obesity, ACPO and ‘other’) found a 24% failure rate, with 3% of those proceeding to intubation. 26% of those started on NIV died in hospital, and only 56% made it out of hospital off NIV (the rest went home on NIV).
So its not a guaranteed win by any stretch and, for some patients, the burden of treatment from repeat blood gases, discomfort of NIV, treatment, monitoring etc may outweigh the possible benefit. It’s not a straightforward intervention, and patients should be involved in decisions about their own treatment. Capacity may fluctuate, and conversations can become complex when family are involved, sedation is considered (eg for agitation), co-morbidities, frailty etc are all thrown into the mix.
In hypercapnic respiratory failure, presenting pH, pH after 1 hour of NIV, severity of disease, and patient co-operation have all been associated with failure of NIV. Failure of NIV thearpy in hypoxic respiratory failure is associated with severity of disease, degree of hypoxia (PaO2/FiO2) and presence of ARDS or pneumonia.
The HACOR score helps predict NIV failure through easy bedside metrics: heart rate, degree of acidosis, conscousness (GCS), oxygenation (PaO2/FiO2), and respiratory rate. Higher scores are associated with higher degrees of NIV failure.
The British Thoracic Society advise that pneumonia, a GCS less than 8, and persisting pH <7.25 or RR >35 all predict NIV failure. To invert this, those who show improvement after 1-2 hours of NIV have a higher predicted rate of successful treatment.
As always, however, there is no single way to predict NIV failure. Clinical judgement in combination with metrics such as those in the HACOR score help to predict success/failure, along with close monitoring of trends in numbers and clinical course after initiation of NIV.
If the patient is showing signs of failure, then early consideration of escalation to invasive ventilation, or palliation should be prioritised.
In summary, factors that may predict NIV failure (in conjunction with clinical assessment):
- Severe acidosis
- Reduced conscious level
- Co-existing pneumonia
- Patient co-operation
- Tachycardia and tachypnoea
- Degree of hypoxia
- Failure to show improvement after 1-2 hours of therapy
- History of NIV failure
Reflecting back to my patient, she presented with severe acidosis, GCS 14, pneumonia, tachycardia and tachypnoea, and failed to show improvement after 1-2 hours of therapy. She would have scored as ‘high risk of failure’ on the HACOR score.
Optimising chances of success
Some points to consider when considering initiating NIV:
- Right patient: ensure that NIV is appropriate. This includes checking against absolute and relative contraindications. If the patient is extremely unwell, should they be immediately escalated to intubation? If not a candidate, should comfort and palliation be a priority?
- Right staff and environment: make sure you and the team are able to manage a patient on NIV, that they get the appropriate close monitoring, ABGs, checks on trends, and that you can troubleshoot problems that arise.
- Patient comms: this is vital. Tell the patient what to expect, that it may feel claustrophobic and uncomfortable, and explain the rationale so that they can at least place the discomfort in context of a life-saving intervention. Consider breaks off the NIV when the patient needs it, but aim to keep on as much as possible for the first 24h. Tell the patient they can signal if any discomfort, concerns or they need to discuss things. Try and put the power of management in their hands and keep them in the loop.
- Adjunctive therapies: continue medical therapies as required such as steroids, bronchodilators, antibiotics for COPD, or GTN, diuretics etc for ACPO. Consider judicious and careful doses of sedatives where indicated for agitation that is compromising treatment (eg low dose lorazepam, Dexmedetomidine in HDU/ITU etc). Any agitation or lack of cooperation warrants proactive team discussion on best interests, capacity and risk:benefit for continuing.
- Have a plan B: you need to consider the patient’s escalation status and what to do next in the event that NIV fails. Have discussions early when patients/NOK are able to, and so preparations (psychological and practical) can be made if things go in the wrong direction. Made simple, would they be for invasive ventilation or palliative care if NIV fails.
References
- Basarik Aydogan, B. (2016) ‘BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults’, Turkish Journal of Medical and Surgical Intensive Care, 7(1), pp. 39–40. doi:10.5152/dcbybd.2016.08.
- Farkas, J. (2023) Noninvasive respiratory support, EMCrit Project. Available at: https://emcrit.org/ibcc/support/ (Accessed: 04 January 2024).
- Ghosh, D. and Elliott, M.W. (2019) ‘Acute non-invasive ventilation – getting it right on the acute medical take’, Clinical Medicine, 19(3), pp. 237–242. doi:10.7861/clinmedicine.19-3-237.
- MacIntyre, N.R. (2019) ‘Physiologic effects of noninvasive ventilation’, Respiratory Care, 64(6), pp. 617–628. doi:10.4187/respcare.06635.
- Mughal, M.M. et al. (2005) ‘Auto-positive end-expiratory pressure: Mechanisms and treatment.’, Cleveland Clinic Journal of Medicine, 72(9), pp. 801–809. doi:10.3949/ccjm.72.9.801.
- P;, N.S. (no date) Causes of failure of noninvasive mechanical ventilation, Respiratory care. Available at: https://pubmed.ncbi.nlm.nih.gov/14982651/ (Accessed: 04 January 2024).
- Yartsev, A. (2021) Non-invasive mechanical ventilation, Deranged Physiology. Available at: https://derangedphysiology.com/main/required-reading/respiratory-medicine-and-ventilation/Chapter%20256/non-invasive-mechanical-ventilation (Accessed: 04 January 2024).